“Internet + Mobile Internet” Intelligent Clinical Trial Cloud Platform
As a full-service CRO, we provide the whole process service of new drug development for biopharmaceutical companies, including scientific consultation, clinical study operation, medical affairs, regulatory affairs, quality management and the 3rd party Audit for Phase I to III studies. Since 2010, more than 200 projects had been operated or are currently on going in CTS. We have been refining in various therapeutic areas, especially in oncology, gastroenterology, CNS, immunology, and respiratory, that we have accumulated significant know-how, capabilities and experiences.
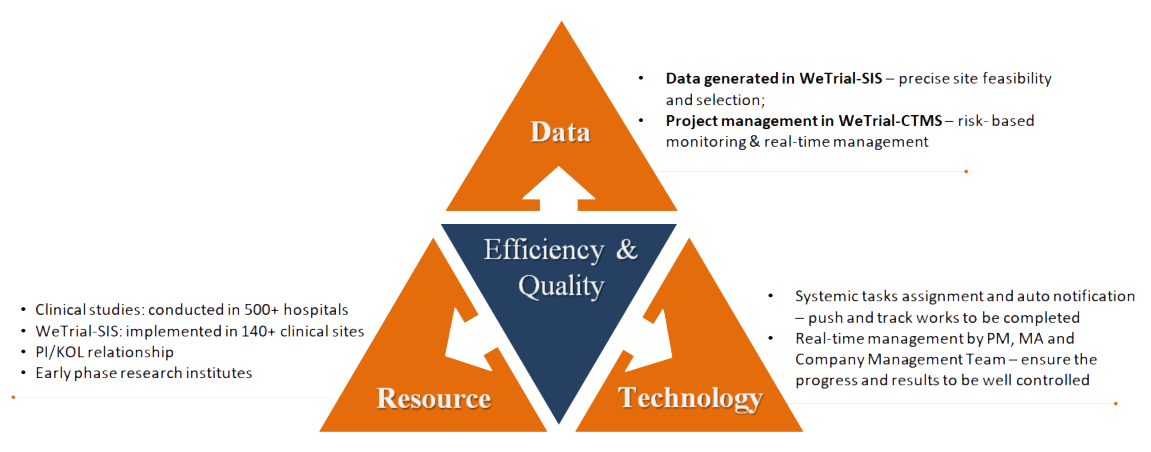
Apart from the regular CRO services, we designed and launched the 1st intelligent clinical trial cloud platform in China linking among all stakeholders to input, analysis, exchange data and information, as well as to manage and communicate progress and risks. That ensures the continuity, traceability and integrity of each data for each project which differentiate CTS from other CROs. From the advantages of having abundant resources with sites and big data generated on this platform, we are able to provide value added services, such as site database for quick SSU, e-patient recruitment for efficient subject enrollment. That speeds up the progress of studies significantly.
Recently, China government encourages more and more hospitals from 2nd/3rd/4th line of city to do clinical trials with required qualifications. We design and provide tailor-made services for these new sites, empower them with SOP establishment and GCP training so as to support them granting the certification of doing clinical trials.